COVID-19 and Regulation
Regulation During a Pandemic
The GW Regulatory Studies Center is actively compiling resources to better understand how COVID-19 is affecting the rulemaking process, and what the US federal government is doing in relation to the pandemic. This page provides resources that we consider helpful in understanding these changes.
COVID-19 Regulations Data Set
This is a supplementary data set to the "2020 Regulatory Year in Review" Insight published by Mark Febrizio and Zhoudan Xie. The list of rules in this data set includes important regulations issued by federal agencies in response to the COVID-19 pandemic in 2020.
Data and Trackers
Skopos Labs
A comprehensive list of bills introduced and passed by Congress and rules introduced and finalized by US federal agencies to address the COVID-19 pandemic.
Skopos Labs is a team of A.I. researchers, data scientists, software engineers, financial professionals, attorneys and policy experts that have developed an automated platform that predicts policy-making outcomes and their impacts on thousands of companies and financial markets. For more information visit their website or email [email protected].
ACAPS COVID-19 Project
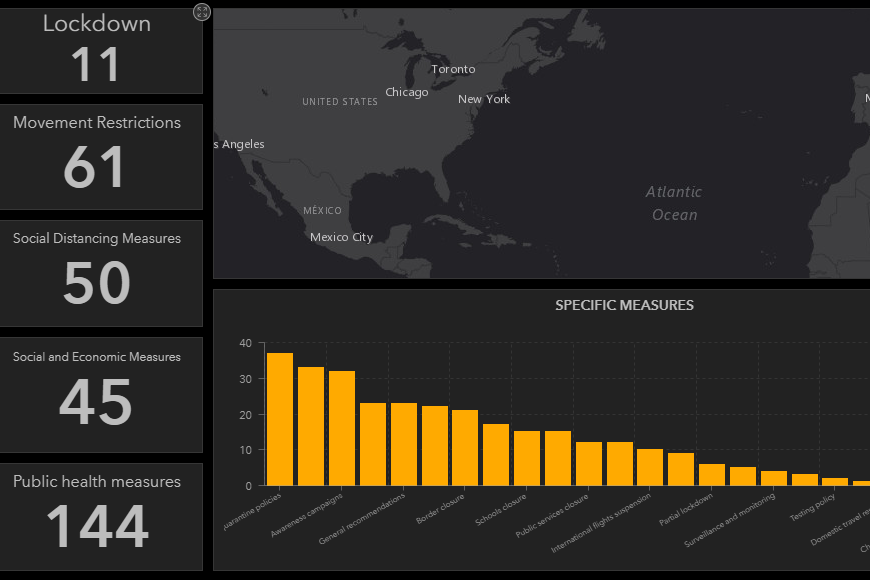
The COVID-19 Government Measures Dataset puts together all the measures implemented by governments worldwide in response to the Coronavirus pandemic. Data collection includes secondary data review. The researched information available falls into five categories: - Social distancing - Movement restrictions - Public health measures - Social and economic measures - Human rights implications Each category is broken down into several types of measures. ACAPS consulted government, media, United Nations, and other organisations sources.
For any comments, please contact them at [email protected]
Additional trackers:
AgencyIQ reviews ClinicalTrials.gov, company announcements, and media reports, for treatment candidates in various stages of testing to assess their potential effects against COVID-19 or SARS-CoV-2, the virus which
causes the condition.
See also:
In late 2019, a new strain of coronavirus emerged in China. With the number of cases of COVID-19, the disease caused by this coronavirus, growing rapidly in the United States and around the world, the World Health Organization declared it a pandemic on March 11, 2020. Controlling the spread of the virus requires aggressive action from states and the federal government to ensure access to testing for those who need it and treatment for those who contract the disease.
To date, states have taken a number of actions to mitigate the spread of the virus and reduce barriers to testing and treatment for those affected. This data tool provides state-level information on:
- Cases and deaths
- Cases and deaths (trend data)
- Adopted social distancing measures
- Health policy actions to reduce barriers to COVID-19 testing and treatment
- Additional state-level data related to COVID-19, including testing and provider capacity
These data will be updated regularly, and new information will be added in response to the evolving situation.
Governments are taking a wide range of measures in response to the COVID-19 outbreak. The Oxford COVID-19 Government Response Tracker (OxCGRT) aims to track and compare government responses to the coronavirus outbreak worldwide rigorously and consistently.
Systematic information on which governments have taken which measures, and when, can help decision-makers and citizens understand the robustness of governmental responses in a consistent way, aiding efforts to fight the pandemic. The OxCGRT systematically collects information on several different common policy responses governments have taken, scores the stringency of such measures, and aggregates these scores into a common Stringency Index.
Data is collected from public sources by a team of dozens of Oxford University students and staff from every part of the world.
NCSL is tracking the impact the coronavirus (COVID-19) may have on state legislatures. Check back often for updated federal agency announcements.
Computational social scientist Rex Douglass provides a dataset compiling restrictions imposed by governments that are mandatory and new (a change to previous rules).
Is there some government regulation or rule that is keeping you from helping manage the COVID-19 crisis?
Maybe you’re a frontline healthcare worker, an administrator, or work in manufacturing, and believe you could make medical supplies. Whatever your position or industry, perhaps you have ideas that could help.
We want to hear from you. Please fill out the form below.
What are the impacts and consequences of the coronavirus pandemic on our lives and our societies – and what are some of the solutions we can find to boost our healthcare systems, secure our businesses, maintain our jobs and education, and stabilise financial markets and economies?
Bloomberg Law provides a comprehensive list of state actions taken in relation to COVID-19.
MultiState Associates provides a useful dashboard of state and local COVID-19 responses.
In furthering Better Markets’ goals of transparency, oversight and accountability, the TRACER is intended to catalogue every coronavirus-related action taken by the financial regulatory agencies:
- Federal Reserve Board (Fed or FRB),
- Securities and Exchange Commission (SEC),
- Commodity Futures Trading Commission (CFTC),
- Office of the Comptroller of the Currency (OCC),
- Federal Deposit Insurance Corporation (FDIC),
- Consumer Financial Protection Bureau (CFPB),
- Financial Stability Oversight Council (FSOC),
- Treasury Department (Treasury), and
- Federal Financial Institutions Examination Council (FFIEC).
The American Action Forum’s COVID-19 Regulation Tracker compiles federal actions related to the coronavirus published in the Federal Register and assigns each a response type. These response types are:
- Finance – Actions related to preservation of markets and liquidity.
- Foreign Entry – Actions related to entering, or reentering, the United States.
- Government – Actions related to the daily functioning of government, including meeting changes, cancellations, comment extensions, and disaster declarations.
- Health – Actions related to increasing medical capacity and preventing the spread of the coronavirus.
- Prohibition – Actions specifically prohibiting a business activity.
- Waiver – Actions granting temporary exemptions from certain regulatory requirements.
- Workforce – Actions related to implementing economic relief to workers, including the Paycheck Protection Program.
This tracker helps you monitor a selection of delayed, repealed, and new rules, notable guidance and policy revocations, and important court battles across eight major categories, including environmental, health, labor, and more.
The Institute for Policy Integrity is tracking altered enforcement of environmental laws by federal and state agencies in response to the COVID-19 pandemic. In connection with the crisis, several agencies have issued waivers or announced plans to stop enforcing key environmental laws and regulations.
Find COVID-19 dental regulations by state with ADA interactive map.
RSC Content on COVID-19 and Regulatory Policy
What are People Saying About COVID-19-Related Regulations?
By: Zhoudan Xie -- July 10, 2020
Government agencies have taken various regulatory actions in response to the COVID-19 pandemic. What are people saying about these regulatory responses? Which ones have prompted the most discussion? And, most importantly, which regulations should be most urgently addressed?
Utilizing ProQuest's TDM Studio, Xie uses text and data mining to gain insights on COVID.
Regulation during COVID-19: News Sentiment Improved, While Uncertainty Remains
By: Zhoudan Xie -- July 6, 2020
This analysis shows that the expression about regulation in the COVID-related news was negative in most days during the beginning of the virus outbreak, but it started to improve in mid-March. However, the level of uncertainty expressed in the news shows no signs of diminishing, indicating persistent uncertainty surrounding regulation in the time of COVID-19.
COVID-19 & CRA Jeopardy
By: Bridget Dooling in Yale JREG -- May 18, 2020
The COVID-19 pandemic has resulted in fewer days in D.C. than usual for Congress. One consequence of this decision is that, due to a quirk in how it’s calculated, the CRA window has likely expanded, placing more rules in jeopardy than expected.
Bring Back the Methadone Vans
By: Laura Stanley in Washington Post -- May 14, 2020
Opioid overdoses are rising. We have an alternative.
For Americans fighting addiction, the pandemic has made life exceptionally challenging. They deserve every tool possible to combat the two crises, including these lifesaving clinics on wheels.
Regulatory Reform to Get the Economy Moving
By: Susan Dudley in Forbes -- May 14, 2020
A dynamic post-pandemic requires thoughtful evaluation to be sure regulations are achieving their goals without needlessly burdening the workers, consumers, small businesses and entrepreneurs who comprise a thriving society. Here are recommendations for emerging from the pandemic with strength.
Regulatory Policy Uncertainty under COVID-19
By: Zhoudan Xie -- May 13, 2020
Uncertainties induced by COVID-19 are worsening the economic impact of the pandemic. Policy uncertainty reached a historical peak during the past two months, while uncertainty specifically around regulatory policy appears to be muted.
Gov in the Time of Coronavirus
By: Susan Dudley in Forbes -- May 8, 2020
The economy may have ground to a halt, but government regulators have not. Not only are they busy issuing new regulations and waiving existing ones to address coronavirus concerns; they are racing against the clock to complete the Trump administration’s regulatory (or deregulatory) priorities
The Federal Government Should Solicit Input From the Public to Help Combat the Coronavirus
By: Daniel R. Pérez in The Hill -- April 8, 2020
Agencies need timely information about where they can look next to provide regulatory relief, and the public is likely to have valuable information to contribute. Every action agencies take represents an opportunity to reduce the spread of this virus—saving hundreds, if not thousands of lives.
Learning From COVID-19
By: Susan Dudley in Forbes -- March 20, 2020
As the world struggles to respond to the COVID-19 pandemic, short-term policies should focus on generating much-needed information. The longer term focus should be on policies that make society more resilient and able to respond to a range of future challenges, whether they are anticipated or not.
The weekly Regulation Digest will continue to provide articles on the intersection of regulation and COVID-19 and other ongoing regulatory developments.
External Commentary & Analysis
Regulatory Provisions in the Phase 3 Stimulus Package
American Action Forum's Dan Bosch and Dan Goldbeck provide analysis of the Coronavirus Aid, Relief, and Economic Security (CARES) Act's regulatory provisions.
To Fight the Coronavirus, Cut the Red Tape
Two economists, Sendhil Mullainathan and Richard H. Thaler, suggest suspending these five types of regulations because they are slowing the medical response to the pandemic.
Timeline: The Regulations—and Regulators—That Delayed Coronavirus Testing
Progressive Policy Institute's Alec Stapp writes about three major regulatory barriers so far.
Read more:
The R Street Institute's Shoshanna Wiessman, Chelsea Boyd, Courtney Joslin, Nick Zaiac, and R. J. Lehmann offer narrow regulatory reforms that could be implemented to help people deal with the effects of COVID-19.
The Mercatus Center at George Mason University is prereleasing a working-paper version of its long-planned 2020 edition of the Healthcare Openness and Access Project (HOAP). It does so in hopes of giving policymakers ideas on how to stretch their healthcare resources as COVID-19 (novel coronavirus) sweeps the country.
Stanford University's John P.A. Ioannidis writes, "Better information is needed to guide decisions and actions of monumental significance and to monitor their impact."
Harvard University's Cass Sunstein argues that "hard-headed cost-benefit analysis usually confirms that it’s dangerous to be overcautious. The coronavirus is different."
Stanford University's Eran Bendavid and Jay Bhattacharya write, 'If it’s true that the novel coronavirus would kill millions without shelter-in-place orders and quarantines, then the extraordinary measures being carried out in cities and states around the country are surely justified. But there’s little evidence to confirm that premise—and projections of the death toll could plausibly be orders of magnitude too high."
Cato Institute's Thomas A. Firey and Peter Van Doren provide lists of rules whose reform would help immediately, and rules whose reform would help in future crises.
Competitive Enterprise Institute's Ryan Young says, "some of the best policy responses are coming not from imposing new regulations, but from loosening old ones."
"There is currently no FDA-approved therapy or vaccine for Covid-19. Given the profound urgency, life sciences companies and other researchers are prioritizing research and development of potential therapies and vaccines."
University of Pennsylvania's Adam Runkle writes, "In the face of a massive viral outbreak, a federal regulator issues unprecedented guidance on validating coronavirus testing."
They were mocked for sounding the alarm. Now they're the ones providing the solutions.
Government’s delay in containing the virus illustrates a fatal weakness of the modern bureaucratic state
Governments are frantically trying to contain and combat the coronavirus, and those efforts are important, but the world’s best hope is private innovation. Cutting-edge diagnostic tests and treatments are advancing, and government should encourage the trend.
President Trump recently ordered the Food and Drug Administration to “slash red tape like nobody’s ever done before” to make medicines approved for other illnesses available for coronavirus patients. The FDA is famously cautious, and safety is important. But drug regulators...
Why would residents block a Covid-19 testing site? For the same reason many oppose other forms of neighborhood change: a desire to shift the burden elsewhere.
By now, readers are aware that testing in the United States for the novel coronavirus COVID-19 has been embarrassingly slow. Less well known is that overregulation is largely to blame. While many news outlets have accurately identified government as the principal cause of delays, reporting on the issue has been woefully incomplete, leaving readers with little understanding of how regulation contributed to the problem. And the coverage seems to have completely missed the fact that the biggest obstacle to more expedient testing has been, ironically, a law intended to expedite treatment during public health emergencies.
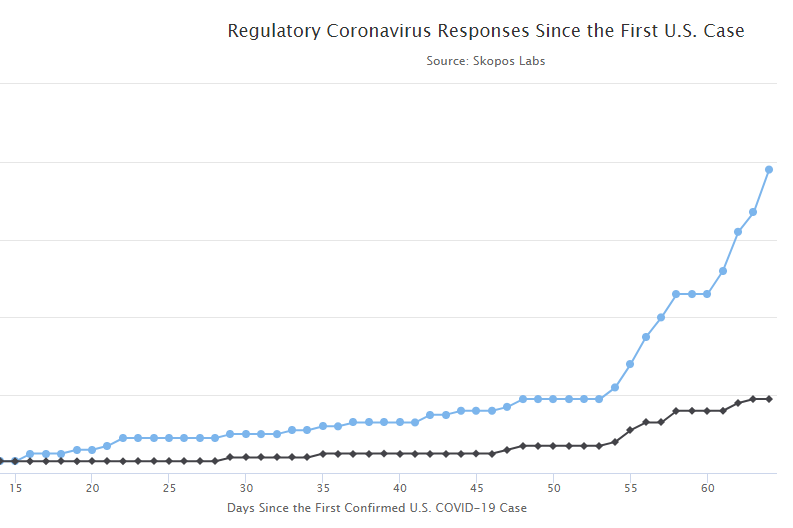
To understand the current crisis requires an understanding of the regulatory and institutional actions taken since January. This chart produced by Senior Research Fellow, Will Rinehart, documents all of the major regulatory changes adopted at the federal level and when they were taken. This timeline shows how and when regulatory agencies at the center of this crisis responded.
This paper develops and implements a method to monetize the impact of moderate social distancing on deaths from COVID-19. Using the Ferguson et al. (2020) simulation model of COVID-19’s spread and mortality impacts in the United States, we project that 3-4 months of moderate distancing beginning in late March 2020 would save 1.7 million lives by October 1. Of the lives saved, 630,000 are due to avoided overwhelming of hospital intensive care units. Using the projected age-specific reductions in death and age-varying estimates of the United States Government’s value of a statistical life, we find that the mortality benefits of social distancing are about $8 trillion or $60,000 per US household. Roughly 90% of the monetized benefits are projected to accrue to people age 50 or older. Overall, the analysis suggests that social distancing initiatives and policies in response to the COVID-19 epidemic have substantial economic benefits.
Forbes contributor John Thorndike recently argued that the coronavirus pandemic could make more Americans inclined to appreciate government and the services it provides.
“It seems quite possible that Americans will emerge from the current crisis with a newfound appreciation for many of the things that government must do — and a keen sense of what happens when it doesn’t do those things or doesn’t do them well,” Thorndike writes.
Yet it’s just as likely that the reverse of what Thorndike posits could also come to fruition. That prospect is evidenced by the more than 110 deregulatory actions taken by federal and state officials across the nation, both Republicans and Democrats, in recent days and weeks.
The FDA has more than 50 regulatory pathways available to companies hoping to get a new drug or biologic approved. Here are the 22 that are most likely to be used to help bring new COVID-19 products to patients.
As part of the war on coronavirus, U.S. regulators are taking aggressive steps against “sludge” – paperwork burdens and bureaucratic obstacles. This new battle front is aimed at eliminating frictions, or administrative barriers, that have been badly hurting doctors, nurses, hospitals, patients, and beneficiaries of essential public and private programs.
Increasingly used in behavioral science, the term sludge refers to everything from form-filling requirements to time spent waiting in line to rules mandating in-person interviews imposed by both private and public sectors. Sometimes those burdens are justified – as, for example, when the Social Security Administration takes steps to ensure that those who receive benefits actually qualify for them. But far too often, sludge is imposed with little thought about its potentially devastating impact.
The coronavirus pandemic is concentrating the bureaucratic mind – and leading to impressive and brisk reforms. Consider a few examples.
Currently, there are “no FDA-approved drugs specifically for the treatment of patients with COVID-19,” per the Centers for Disease Control and Prevention. There is also no vaccine. But there are antiviral drugs that show promise in tests and actual use. Food and Drug Administration emergency use powers should be exercised to fast-track the approval and use of these drugs. Confidence in accelerated approval will facilitate greater speed in their manufacture, which can and should be assured by the federal government through use of the Defense Production Act.
Leading scientists have predicted dire short-term public health impacts from the spread of the coronavirus, and the resulting COVID-19 disease. The president has said holding U.S. deaths under 100,000 would reflect good efforts. Other estimates are far higher. A reasonable weighing of risks and benefits shows that quick testing, approval and distribution of promising drugs could save hundreds of thousands of lives and help to mitigate the current medical care crisis.
As of March 20, at least 17 different drugs are in various states of development and clinical trials as potential treatments for COVID-19. These include Gilead’s Remdesivir, which seeks to interrupt the coronavirus’ ability to replicate and is subject to various clinical tests. Roche’s Actemra was approved in China for use of treatment of severe complications related to the coronavirus. And beginning March 24, New York started a clinical study of hydroxychloroquine and azithromycin, which showed promise in a small French study.
In public health practice, “quarantine” refers to the separation of persons (or communities) who have been exposed to an infectious disease. “Isolation,” in contrast, applies to the separation of persons who are known to be infected. In U.S. law, however, “quarantine” often refers to both types of interventions, as well as to limits on travel. Isolation and quarantine can be voluntary or imposed by law.
Inside the country, isolation and quarantine orders have traditionally come from the states. Courts have typically upheld these orders in deference to the states’ broad powers to protect public health. Nevertheless, courts have occasionally intervened when a quarantine was unreasonable or when officials failed to follow necessary procedures. For example, in Jew Ho v. Williamson (1900), a federal court struck down a quarantine imposed by San Francisco in response to an outbreak of bubonic plague because it was racially motivated and ill-suited to stop the outbreak.
Throughout the response to the COVID19 crisis, many healthcare institutions have increased their use of telecommunications. From medical schools to residency programs, from patient interactions at home to those in quarantine, virtual communication is providing a safe way to continue with our responsibilities during this pandemic. According to POTUS (17 March 2020), telemedicine, with lessened HIPAA regulations, is now an approved method of communication between physicians and patients for those with Medicare. Telemedicine provides protection to both the physician and patient, preventing possible spread of COVID-19 while allowing for continued patient care. It is now vital to make practical, effective use of telecommunications to stabilize the healthcare system.
Leading scholars from around the world discuss the administrative law and regulatory dimensions to the global response to COVID-19.
The Facebook page that called for Egyptians to take to the streets on January 25, 2011—a day that would prove pivotal to the Arab Spring uprisings—was almost relegated to history. Just a few short months prior, Facebook had taken the page down, citing its policy requiring people to use their real name on the platform.
There has been plenty of talk about how the pandemic demonstrates the need for more government, but the advantages that can come from regulatory relaxation suggest that, on the contrary, it has made the case for rather less.
Contact tracing for COVID-19 is a necessary tool to allow communities to reopen. Unfortunately, because of the speed and numbers of COVID-19 cases, manual contact tracing is unlikely to be sufficient. Digital contact tracing can provide enough capacity but comes with serious privacy concerns.
After many used medical syringes washed up on beaches in the eastern United States, Congress enacted the Medical Waste Tracking Act of 1988, which directed EPA to establish a two-year medical waste demonstration project that would principally affect several northeastern states.
The innovation we have seen during the crisis should help guide regulatory reform efforts in the future.
We need to update alcohol regulation and treat it more like other products. Delivery and to-go restrictions are remnants of a Prohibition mentality.
The Trump Administration’s long parade of deregulation—on everything from Title IX, to net neutrality, to environmental-impact statements, to joint employers—is among its biggest achievements. Amid the coronavirus pandemic, this work has thankfully continued.
How have regulations stymied the response to the COVID-19 pandemic? And what explains the intense regulatory scrutiny tech companies face?
Yesterday the White House issued a new executive order titled “Executive Order on Regulatory Relief to Support Economic Recovery.” It is intended, most immediately and obviously, to amplify the economic recovery so sorely needed amid the Covid-19 crisis. But President Trump’s order could have significant long-term effects, because it contains what we can think of as “pilot projects” or “case studies” for broader and more fundamental administrative reforms.
If Congress must do something in the next few weeks, they would be wise to fix all these regulatory issues that will delay a return employment rather than spend more money.
The guidance, intended to support companies racing to develop treatments for the novel coronavirus, outlines best practices for designing clinical trials that the FDA will consider sufficient to support approval. However, the guidance also notes several areas in which the agency is not yet providing recommendations, indicating that changes are yet to come.
A streamlined government would be a godsend to marshal resources for social needs and to liberate human initiative at every level of society.
At the beginning of 2017, the Trump Administration committed to reducing federal regulation and reining in regulatory costs. In fact, Executive Order 13771 was filed on January 20th, 2017 to do just that. Since that time, there has been a significant amount of deregulation across policy arenas such as the environment, labor, and health care. However, the SARS-CoV-2 coronavirus has infiltrated the legislative and regulatory world – just as it has every other facet of life.
The Trump administration has undertaken a series of deregulatory measures to address various challenges of the COVID-19 pandemic. The Brookings’ Center on Regulation and Markets is actively tracking these actions alongside the administration’s broader deregulatory agenda. We asked scholars from the Brookings Economic Studies Program for their thoughts on some of the most impactful COVID-related deregulations to date.
Popular News Reports
The U.S. is relaxing rules for medical professionals working across state lines
NPR - Marketplace’s senior economics contributor Chris Farrell explains how states are making changes.
U.S. Suspends Truck-Driving Limits to Speed Coronavirus Shipments
Wall Street Journal - The move comes as hospitals report shortages and retailers and manufacturers strain under surging demand
COVID-19 testing delays shine light on lab-developed test regulation debate
Modern Healthcare - This month, lawmakers introduced a bill that would vastly change the oversight system for diagnostics in this country and give the U.S. Food and Drug Administration explicit authority to regulate tests developed by labs.
Read more:
The federal government announced Monday that it was relaxing many of its usual safety standards for hospitals so they could expand services to fight the coronavirus pandemic.
The Centers for Medicare and Medicaid Services is changing rules on what counts as a hospital bed; how closely certain medical professionals need to be supervised; and what kinds of health care can be delivered at home. These broad but temporary changes will last the length of the national emergency.
Designating pandemic a ‘major disaster’ would increase assistance, but virus is not listed as a qualifying natural disaster under the law
Co-Diagnostics says it has capacity to ‘supply all of the testing needs in Utah and around us easily’
As much of his government battles the coronavirus outbreak, President Trump is pushing ahead with major reversals of environmental regulations, including a restriction on scientific research that some doctors worry would complicate future pandemic controls.
Federal employees across multiple agencies said the administration was racing to complete a half-dozen significant rollbacks over the coming month. They include a measure to weaken automobile fuel efficiency standards, which one person familiar with the plans said would be issued as early as next week.
Environmentalists are urging the Interior Department to hit the pause button on policy decisions — from public comment periods to oil and gas lease sales — as the nation attempts to address the coronavirus pandemic, but the Trump administration has shown no indication it plans to do so.
A federal agency’s decision to allow lab companies to release coronavirus tests without prior government approval should help ease the shortage of test kits, but at the potential cost of compromised results, medical experts said Tuesday.
“It’s a pretty good idea to allow for companies to get tests out in a national emergency,” said Paul Fey, research medical director at the University of Nebraska Medical Center. But he added that “these tests may not perform as well.”
Regulators are rushing to finish work as soon as next week on some top Trump initiatives, including relaxing fuel economy standards for vehicles and eliminating the legal basis for restricting mercury pollution from coal-fired power plants. The haste is necessary to prevent the measures from being killed by Congress next year if President Donald Trump loses re-election and Democrats retake the Senate.
U.S. regulators gave banks a reprieve from new accounting standards that require lenders to book losses on soured loans more quickly, the latest step designed to encourage banks to keep lending during the spread of the new coronavirus.
Banks now have up to two additional years before they must set aside more capital for reserves against loan losses, as required by the new “current expected credit loss,” or CECL, accounting standard.
Banks should re-enter the business of offering short-term, small-dollar loans to cash strapped-customers in light of the outbreak of the coronavirus, a group of regulators said on Thursday.
The move, announced in a joint statement of the Federal Deposit Insurance Corp. and a group of other regulators, marks an effort to revive a riskier lending sector that dried up in the years after the 2008 financial crisis. In encouraging financial firms to move back into this market, regulators say they hope to steer consumers away from more-predatory, payday forms of lending.
The Environmental Protection Agency on Thursday announced a sweeping relaxation of environmental rules in response to the coronavirus pandemic, allowing power plants, factories and other facilities to determine for themselves if they are able to meet legal requirements on reporting air and water pollution.
The move comes amid an influx of requests from businesses for a relaxation of regulations as they face layoffs, personnel restrictions and other problems related to the coronavirus outbreak.
Early on, the dozen federal officials charged with defending America against the coronavirus gathered day after day in the White House Situation Room, consumed by crises. They grappled with how to evacuate the United States consulate in Wuhan, China, ban Chinese travelers and extract Americans from the Diamond Princess and other cruise ships.
The members of the coronavirus task force typically devoted only five or 10 minutes, often at the end of contentious meetings, to talk about testing, several participants recalled. The Centers for Disease Control and Prevention, its leaders assured the others, had developed a diagnostic model that would be rolled out quickly as a first step.
The federal government announced Monday that it was relaxing many of its usual safety standards for hospitals so they could expand services to fight the coronavirus pandemic.
The Centers for Medicare and Medicaid Services is changing rules on what counts as a hospital bed; how closely certain medical professionals need to be supervised; and what kinds of health care can be delivered at home. These broad but temporary changes will last the length of the national emergency.
As the number of COVID-19 cases increase in the US and hospitals struggle with keeping their doctors and nurses protected, the Food and Drug Administration (FDA) on Monday released guidance explaining how it will relax certain regulatory requirements to increase the production and availability of certain personal protective equipment (PPE) such as gowns and gloves.
As early as late February, FDA began warning of PPE shortages, and hospitals in areas hit hard by the coronavirus are now seeing the reuse of some single-use masks and other issues.
States have enacted a wide range of policies in an effort to "flatten the curve" and address the economic effects of the novel coronavirus. While all 50 states have declared states of emergency, giving governors emergency powers, the policy response from states has been wide-ranging.
President Donald Trump signed an executive order Tuesday directing federal agencies to ease up on businesses that make good-faith attempts to follow agency guidance and regulations during the coronavirus pandemic.