Download this commentary (PDF)
In brief ...
Amid a global trend of exempting regulations from the requirement to conduct regulatory impact analyses, the OECD points out the inherent conflict: while exemptions can streamline the regulatory process by reducing costs and avoiding delays, this benefit comes at the expense of potentially undermining the effectiveness of the regulations themselves. This commentary assesses actions taken by the Brazilian Health Risk Regulatory Agency (ANVISA) regarding RIA exemptions.
In a recent study published in the Journal of Benefit-Cost Analysis, we examine the behavior of the Brazilian Health Risk Regulatory Agency (ANVISA) regarding exemptions from the requirement for Regulatory Impact Analysis (RIA), particularly in emergency situations. Decisions to grant exemptions are critical for the institutionalization of RIA in Brazil, and understanding this trend is vital for improving regulatory practices nationally and globally, as similar patterns are emerging internationally.
The Growth of Exception Decisions Is a Global Trend
The global trend of exempting regulations from the requirement to conduct RIAs, especially during emergencies, is a significant concern. The Organization for Economic Co-operation and Development (OECD) identified this as a problem in its 2021 Regulatory Policy Outlook, highlighting a growing trend among its member countries in excusing RIA requirements due to emergencies. The COVID-19 pandemic amplified this phenomenon, so that in 2020, a total of 18 OECD member countries did not require RIAs when implementing emergency-related regulations. This represents a 38% increase in member countries in this situation over the three years from 2017.
The OECD points out the inherent conflict: while exemptions can streamline the regulatory process by reducing costs and avoiding delays, this benefit comes at the expense of potentially undermining the effectiveness of the regulations themselves. This is because the lack of impact studies associated with RIAs can hinder the ability to serve the public interest through regulation.
Furthermore, the lack of transparency and scrutiny surrounding these exemption decisions allows for their potential misuse. Regulators may strategically cite emergencies to justify exemptions, even when the situation does not fully warrant it. This, in turn, shadows the underlying preferences and value judgments influencing the decisions. The established importance of RIAs in promoting rational, evidence-based decision-making and ensuring bureaucratic accountability (De Francesco and Radaelli 2007) is clearly compromised.
While some OECD countries have adopted post-implementation assessments as a way to mitigate these issues, this solution has challenges. The quality of these assessments depends heavily on the availability of information about the underlying problems and objectives of the regulation, and this information is often missing when an RIA has not been conducted. Therefore, even this corrective measure is limited in its effectiveness. The OECD recognizes the need for flexibility in applying RIAs, particularly in true emergencies, but a balance must be struck to ensure both efficiency and accountability in the regulatory process. The ever-increasing use of full exemptions indicates a worrying trend toward routine circumvention of the important checks and balances afforded by RIAs.
Exception Decisions in Brazil Before and After Presidential Order No. 10,411/2020
High rates of RIA exemptions due to emergencies have long characterized Brazilian regulatory practice. Even before the COVID-19 pandemic, as our previous study (Salinas & Gomes, 2021) has found, ANVISA exempted from the RIA requirement 56.7% (478 of 843) of its regulations approved between 2012 and 2020, citing urgency in 86.2% (412 of 478) of those cases. This resulted in the omission of RIAs for 48.9% of new regulations.
The subsequent Presidential Order No. 10,411/2020 (effective April 15, 2021) expressly outlined new situations, not anticipated in ANVISA’s previous rules, in which the RIA requirement could be ignored at the discretion of regulatory agencies. In addition to emergencies, which had previously been qualified as a cause for exemptions, the Order included new exceptions for completing RIAs, including low-impact rules, rules complying with international standards, rules reducing regulatory costs, and rules lessening administrative burdens on regulated entities.
Furthermore, the Order has also introduced additional cases not subject to discretion, where RIA must not be applied (inapplicability cases), such as when a new rule is merely consolidating previous rules (text consolidation). In exemption cases, the agency has to offer a justification, outlining the reasons that led to the exception decision. In cases of inapplicability, there is no such requirement. Furthermore, the Order mandates post-implementation evaluations within three years of any emergency-related RIA exemption (Article 4, Section 2), following OECD guidelines favoring ex-post reviews in such instances.
Many of these inapplicability and exemptions causes have no parallel in other countries. They create significant ambiguity and allow considerable agency discretion, undermining the intended goals of the RIA process and diverging from OECD best practices.
Less RIAs, Further Excuses
Our new study analyzes ANVISA regulations approved after Presidential Order No. 10,411/2020, examining the agency's justifications for RIA exemptions. We have assessed changes in RIA usage, the types of justifications used for exemptions, and ANVISA's handling of emergency exemptions.
Our findings have shown a considerable decrease in ANVISA's usage of RIAs following the implementation of the Order on April 15, 2021. Prior to this date, RIAs were used in approximately one-third (33.67%) of the 879 regulations issued since January 2011. However, this dropped to roughly one-tenth (10.38%) of the 395 regulations issued between April 15, 2021, and December 2022 (see Graph 1).
Graph 1

A longer-term perspective reveals a steadily declining trend in RIA usage, with annual percentages decreasing from 36.54% in 2019 to 20.13% in 2020, 17.47% in 2021, and a mere 8.3% in 2022. This demonstrates a significant and sustained decrease in the use of RIAs.
Graph 2

While the substantial increase in rulemaking, particularly concerning regulatory stock consolidation, partly explains the sharp decrease in RIA usage in 2022 (frequently cited as the reason for inapplicability on the agency website), a significant decline remains even after accounting for this factor. Even with inapplicability cases excluded, the 2022 RIA usage rate of 16.54% is considerably lower than previous years, indicating a persistent downward trend.
Analysis of the justifications for RIA exemptions reveals a significant shift following the implementation of Presidential Order No. 10,411/2020. Before the Order, "urgency/emergency" had overwhelmingly dominated justifications. However, the introduction of additional justifications resulted in a diversification of reasons provided for exemption, with "urgency/emergency" becoming far less prevalent (see Table 1 for details).
Table 1
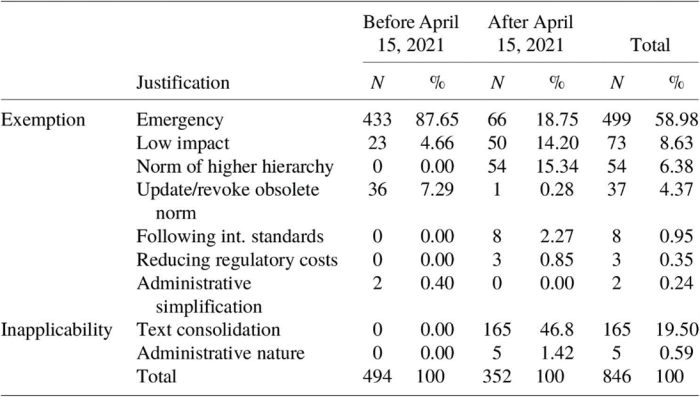
A Lack of Clear Criteria to Substantiate Exemption Cases
Our new study has included a qualitative analysis of post-April 15, 2021 justification documents for urgency/emergency exemptions. Two categories of exempted regulations emerged: (i) those directly related to COVID-19 (65.2%), and (ii) those unrelated to the pandemic (34.8%).
Our analysis revealed a lack of demonstrated urgency and rigorous emergency definitions, particularly in non-COVID-19 related exemptions. Many of these regulations seem to qualify for low-impact exemptions, highlighting a need for clearer methodologies (e.g., proportionality requirements and threshold tests) within the Brazilian context to define low impact.
While low-impact regulations may not require RIAs, avoiding unnecessary constraints on regulatory choices, the absence of clear parameters defining "low impact" risks omitting RIAs in cases where they are crucial.
A notable aspect of the COVID-19 related exemptions involves ANVISA's adoption of temporary emergency regulations during the pandemic. While Presidential Order No. 10,411/2020 mandated ex-post evaluations within three-year approval of such regulations, ANVISA's subsequent Ordinance No. 162/2021 (Article 57, §2) removed this requirement for temporary regulations, encompassing the majority of COVID-19 related measures.
ANVISA's decision to exempt temporary emergency regulations from ex-post evaluation, while not yet judicially reviewed, is problematic. First, the effects of temporary regulations often extend beyond their stated duration. Second, the increasing frequency of health crises demands the evaluation of such measures for potential future application.
The Need for Further Critical Analysis of Exception Decisions
This study highlights the critical role of RIA exemptions in the effectiveness of Brazilian regulatory policy. The ambiguities within Presidential Order No. 10,411/2020 hinder the proper application of RIAs, emphasizing the need for clearer criteria balancing the need for rapid responses with thorough impact analysis. While the legal framework may differ internationally, the increasing use of exemptions for RIA requirements during emergencies presents similar challenges requiring further research and attention globally.

